Products Details

Application: it has obvious liver protection effect, suitable for acute and chronic hepatitis, early cirrhosis, etc
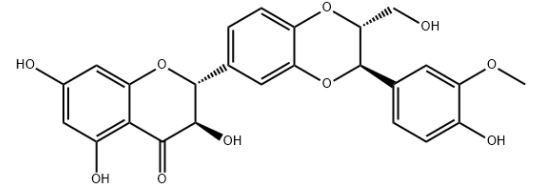
Synonyms:SILYBIN;SILYBIN A;SILYBIN (A AND B);SILYMARIN;SILYMARIN I;SILYBININ;SILYBIN (MIX);SILIBININ
CAS:22888-70-6
MF:C25H22O10
MW:482.44
EINECS:245-302-5
Product Categories:Aromatics;Chiral Reagents;Angiogenesis InhibitorsCancer Research;Anti-proliferative AgentsNutrition Research;Angiogenesis;Biochemicals Found in Plants;Bioflavonoids;Cancer Research;Chemopreventive Agents;Natural Plant Extract;Flavanones;Inhibitors;Plant extract;chemical reagent;pharmaceutical intermediate;phytochemical;reference standards from Chinese medicinal herbs (TCM).;standardized herbal extract;Plant extracts;Pharmaceutical raw material;Intermediates & Fine Chemicals;Pharmaceuticals
Silibinin Chemical Properties
Melting point 164-174°C
alpha D20 +11° (c = 0.25 in acetone + alcohol)
Boiling point 793.0±60.0 °C(Predicted)
density 1.527±0.06 g/cm3(Predicted)
storage temp. ?20°C
pkapKa 6.42±0.04 (Uncertain)
Water Solubility 54mg/L(24.99 oC)
Merck 13,8607
Stability:Stable. Incompatible with strong oxidizing agents, strong bases.
CAS DataBase Reference22888-70-6(CAS DataBase Reference)
Safety Information
Hazard Codes Xi
Risk Statements 36/37/38
Safety Statements 26-37/39-24/25-22-36
RIDADR 3172
WGK Germany 3
RTECS DJ2981770
HS Code 29329990
ToxicityLD50 intravenous in mouse: 1056mg/kg
MSDS Information
Silibinin Usage And Synthesis
Chemical Propertiessolid
OriginatorLegalon,Madaus,W. Germany,1969
UsesLabelled Silybin (S465850). Hepatoprotectant.
DefinitionChEBI: A flavonolignan isolated from milk thistle, Silybum marianum, that has been shown to exhibit antioxidant and antineoplastic activities.
Manufacturing ProcessSilymarin comprising polyhydroxyphenyl chromanones is recovered from the dried fruit of Silybum marianum Gaertn. by separating the fatty oils therefrom, extracting the remaining solid residue with ethyl acetate, evaporating the ethyl acetate and dissolving the dry residue in a solvent mixture comprising methanol, water and petroleum ether to form a two-phase system wherein the chromanones are contained in the lower phase, recovering the polyhydroxyphenyl chromanones from the lower phase after subjecting same to multiple counter-current contact with petroleum ether.
Therapeutic FunctionHepatoprotectant
Displaying 1 to 3 (of 6 products)