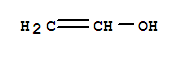Products Details

Poly(vinyl alcohol) 9002-89-5 manufacturer
Poly(vinyl alcohol) 9002-89-5 price
Poly(vinyl alcohol) 9002-89-5 in stock
Physicochemical properties White to cream-colored granules or powder
Application: used as warp size, emulsion stabilizer, rewetting adhesive, water soluble film, etc
Poly(vinyl alcohol) Usage And Synthesis
Air & Water ReactionsWater soluble.
Fire Hazard:This chemical is combustible. The dusts of this chemical are a slight explosion hazard when exposed to flame. (NTP, 1992)
Health HazardSYMPTOMS: Inhalation of the dust of this chemical may cause irritation of the nose and throat and cause coughing and chest discomfort if heated above 390° F. The dusts may also irritate the eyes. Implantation of this chemical into the breast has been associated with fibrosis. ACUTE/CHRONIC HAZARDS: This compound may be harmful by ingestion and inhalation. It may cause irritation. When heated to decomposition it emits acrid smoke, irritating fumes and toxic fumes of carbon monoxide and carbon dioxide. (NTP, 1992)
Reactivity ProfileMixtures of alcohols with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions. Example: an explosion will occur if dimethylbenzylcarbinol is added to 90% hydrogen peroxide then acidified with concentrated sulfuric acid. Mixtures of ethyl alcohol with concentrated hydrogen peroxide form powerful explosives. Mixtures of hydrogen peroxide and 1-phenyl-2-methyl propyl alcohol tend to explode if acidified with 70% sulfuric acid [Chem. Eng. News 45(43):73. 1967; J, Org. Chem. 28:1893. 1963]. Alkyl hypochlorites are violently explosive. They are readily obtained by reacting hypochlorous acid and alcohols either in aqueous solution or mixed aqueous- carbon tetrachloride solutions. Chlorine plus alcohols would similarly yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M 1991]. Base-catalysed reactions of isocyanates with alcohols should be carried out in inert solvents. Such reactions in the absence of solvents often occur with explosive violence [Wischmeyer 1969].
Chemical propertiesPolyvinyl alcohol is a hydrolysis product of polyvinyl acetate, rather than by the polymerization of monomers; the molecular backbone contains . The specific gravity of this product 1.25 to 1.35 and the melting point is 212 ~ 267 ℃. It is soluble in hot water and hot dimethyl sulfoxide. Animal experiments show that polyvinyl alcohol, without stimulation, causes no significant toxicity upon subcutaneous, intramuscular, intravenous injection. Polyvinyl alcohol resin products appear as white solid with the appearance of sub-floc, granular and powder; it is non-toxic, tasteless, non-polluting and is soluble in water of 80--90 ℃. Its aqueous solution has good adhesiveness and film-forming property; it can resist most organic solvents such as oils, lubricants and hydrocarbons; it has long-chain polyol esterification, etherification, acetalization and other chemical properties.
Uses:It is mainly used in the textile industry, as the raw materials of warp pulp, fabric finishing agent, vinylon fiber; interior and exterior wall paint of the building, adhesives; chemical industry use it as a polymerization emulsifier, dispersant and polyvinyl formal, acetal, butyrate aldehyde resin; paper industry use it as a paper binder; agriculture use it as soil improvers, pesticide adhesion synergist and polyvinyl alcohol film; it can also be used for daily cosmetics and high-frequency quenching agent and so on.
Chemical Properties:white or cream solid
Chemical Properties:Polyvinyl alcohol occurs as an odorless, white to cream-colored granular powder.
Uses:In the plastics industry in molding Compounds, surface coatings, films resistant to gasoline, textile sizes and finishing compositions; can be compounded to yield elastomers to be used in manufacture of artificial sponges, fuel hoses, etc., also in printing inks for plastics and glass, in pharmaceutical finishing, cosmetics, water-sol film and sheeting. Pharmaceutic aid (viscosity increasing agent); ophthalmic lubricant.
DefinitionChEBI: A homopolymer macromolecule obtained by polymerisation of vinyl alcohol.
Production Methods:Polyvinyl alcohol is produced through the hydrolysis of polyvinyl acetate. The repeating unit of vinyl alcohol is not used as the starting material because it cannot be obtained in the quantities and purity required for polymerization purposes. The hydrolysis proceeds rapidly in methanol, ethanol, or a mixture of alcohol and methyl acetate, using alkalis or mineral acids as catalysts.
Brand name:Liquifilm Tears (Allergan).
Pharmaceutical Applications:Polyvinyl alcohol is used primarily in topical pharmaceutical and ophthalmic formulations. It is used as a stabilizing agent for emulsions (0.25–3.0% w/v). Polyvinyl alcohol is also used as a viscosity-increasing agent for viscous formulations such as ophthalmic products. It is used in artificial tears and contact lens solutions for lubrication purposes, in sustained-release formulations for oral administration, and in transdermal patches. Polyvinyl alcohol may be made into microspheres when mixed with a glutaraldehyde solution.
Industrial uses:Polyvinyl alcohol is a tough, whitish polymerthat can be formed into strong films, tubes, andfibers that are highly resistant to hydrocarbonsolvents. Although polyvinyl alcohol is one ofthe few water-soluble polymers, it can be renderedinsoluble in water by drawing or by theuse of cross-linking agents.
Safety ProfileQuestionable carcinogen with experimental carcinogenic and tumorigenic data by implant route. Flammable when exposed to heat or flame; can react with oxidizing materials. Slight explosion hazard in the form of dust when exposed to flame. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety:Polyvinyl alcohol is generally considered a nontoxic material. It is nonirritant to the skin and eyes at concentrations up to 10%; concentrations up to 7% are used in cosmetics.
Studies in rats have shown that polyvinyl alcohol 5% w/v aqueous solution injected subcutaneously can cause anemia and infiltrate various organs and tissues.
(mouse, oral): 14.7 g/kg
(rat, oral): >20 g/kg
storage:Polyvinyl alcohol is stable when stored in a tightly sealed container in a cool, dry place. Aqueous solutions are stable in corrosionresistant sealed containers. Preservatives may be added to the solution if extended storage is required. Polyvinyl alcohol undergoes slow degradation at 100°C and rapid degradation at 200°C; it is stable on exposure to light.
Incompatibilities:Polyvinyl alcohol undergoes reactions typical of a compound with secondary hydroxy groups, such as esterification. It decomposes in strong acids, and softens or dissolves in weak acids and alkalis. It is incompatible at high concentration with inorganic salts, especially sulfates and phosphates; precipitation of polyvinyl alcohol 5% w/v can be caused by phosphates. Gelling of polyvinyl alcohol solution may occur if borax is present.
Regulatory StatusIncluded in the FDA Inactive Ingredients Database (ophthalmic preparations and oral tablets). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Displaying 1 to 3 (of 6 products)