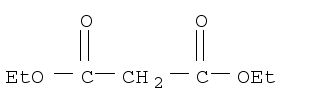Diethyl malonate 105-53-3 manufacturer
Diethyl malonate 105-53-3 price
Diethyl malonate 105-53-3 in stock
Physicochemical properties: colorless liquid with sweet ether smell.
Melting point - 50 ℃
Boiling point 199.3 ℃
The relative density was 1.0551
Refractive index 1.4135
Flash point 100 ℃
It is miscible with alcohol and ether, soluble in chloroform, benzene and other organic solvents. Slightly soluble in water. The solubility in water is 2.08g/100ml at 20 ℃
Uses: used as intermediates of pharmaceutical weekly sulfonamides and barbiturates, as well as intermediates of spices and dyes
Diethyl malonate Usage And Synthesis
Description:As an organic compound, diethyl malonate belongs to the diethyl ester of malonic acid, which is present naturally in guava fruits, melons, grapes, pineapples, blackberries and strawberries as a colorless liquid with an apple-like odor. It is a flavor ingredient commonly found in perfumes, artificial flavorings, alcoholic beverages, various wines and spirits due to its natural pleasant odor. It is also used as an essential intermediate in the syntheses of numerous pharmaceuticals, such as barbiturates, vitamins B1 and B6, non-steroidal anti-inflammatory agents. Besides, diethyl malonate is also involved in organic synthesis of other compounds, such as alpha-aryl malonates, mono-substituted and di-substituted acetic acid. And it can react with benzaldehyde for the production of diethyl benzylidenemalonate in Knoevenagel condensation reaction.
References:https://en.wikipedia.org/wiki/Diethyl_malonate
https://pubchem.ncbi.nlm.nih.gov/compound/7761#section=Safety-and-Hazards
https://www.alfa.com/zh-cn/catalog/A15468/
http://www.hmdb.ca/metabolites/HMDB29573
http://www.chemicalland21.com/industrialchem/organic/DIETHYL%20MALONATE.htm
Chemical Propertiescolourless liquid
Chemical PropertiesDiethyl malonate has a faint, pleasant, aromatic odor.
Occurrence:Reported found in pineapple, bilberry, Cape gooseberry, cognac, malt whiskey, apple brandy, grape brandy, port, cider, sherry and red, white, strawberry and bilberry wines.
Uses:Diethyl Malonate occurs naturally in grapes and strawberries. It is used in the preparation of barbiturates, artificial flavourings, vitamin B1, and vitamin B6 as well as in perfumes.
Uses:manufacture of barbiturates.
Preparation:Reacting chloroacetic acid to cyanoacetic acid using sodium cyanide and subsequent saponification; malonic acid is finally esterified by azeotropic distillation with ethanol in benzene
Taste threshold values:Taste characteristics at 50 ppm: sweet and fruity with apple and pineapple nuances.
Synthesis Reference(s):The Journal of Organic Chemistry, 46, p. 3151, 1981 DOI: 10.1021/jo00328a041
Synthesis, p. 59, 1987
Tetrahedron Letters, 36, p. 3997, 1995
Safety Profile:Mildly toxic by ingestion. A skin irritant. Combustible liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use water to blanket fire, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
Metabolism:When the ester was fed to chicks at a level of 5% in the diet, 32% of the energy from diethyl malonate was available (Yoshida et al. 1970). Hydrolysis of diethyl malonate would produce ethanol and malonic acid, which is a relatively strong acid and acts as an inhibitor of enzymes, including succinic dehydrogenase (Fassett, 1963). Malonic acid injected into rats or rabbits is excreted largely unchanged, but also causes increased excretion of citric and a-ketoglutaric acids (Krebs, Salvin & Johnson, 1938). Some malonate may be metabolized through the tricarboxylic acid cycle, with decarboxylation to acetate followed by transformation to succinate, which has been detected in rat urine (Lee & Lifson, 1951). Diethyl malonate was hydrolysed by adipose-tissue lipase (Lynn & Perryman, 1960) and to the monoester by α-chymotrypsin (Cohen & Crossely, 1964). It was oxidized in 110 min to the extent of 34% by the homogenized mycelium of urethane-grown Streptomyces nitrifica (Schatz, Trelawny, Schatz & Mohan, 1957).
Purification Methods:If too impure (IR, NMR) the ester (250g) is heated on a steam bath for 36hours with absolute EtOH (125mL) and conc H2SO4 (75mL), then fractionally distilled under reduced pressure. Otherwise fractionally distil it under reduced pressure and collect the steady boiling middle fraction. [Beilstein 2 IV 1881.]
Diethyl malonate Preparation Products And Raw materials
Raw materials:Ethanol-->Hydrochloric acid-->Sulfuric acid-->Sodium carbonate-->Sodium cyanide-->Malonic acid-->Chloroacetic acid-->Chloroacetic acid sodium salt-->Cyanoacetic acid-->Sodium Phosphate, Dibasic-->MALONIC ACID DISODIUM SALT
Preparation Products:2-HEXYLDECANOIC ACID-->CYCLOPENTYLACETIC ACID-->5-CHLORO-BENZOFURAN-2-CARBOXYLIC ACID ETHYL ESTER-->Enoxacin-->Phenylbutazone-->4,6-Dichloro-2-(methylsulfonyl)pyrimidine-->2-Benzofurancarboxylic acid ethyl ester-->5-NITROBENZOFURAN-2-CARBOXYLIC ACID-->4-CHLORO-2-METHANESULFONYL-6-METHOXY-PYRIMIDINE-->Diethyl chloromalonate-->ETHYL 7-METHOXYBENZOFURAN-2-CARBOXYLATE-->5,7-BIS(TRIFLUOROMETHYL)-4-CHLOROQUINOLINE-->2-Mercapto-4,6-dimethoxypyrimidine-->4-Chloro-6-methoxy-2-(methylthio)pyrimidine ,98%-->5,7-BIS(TRIFLUOROMETHYL)-4-HYDROXYQUINOLINE-->2-(TRIFLUOROMETHYL)-4-HYDROXYPYRIMIDINE-5-CARBOXYLIC ACID-->5-Nitropyridine-2-carboxylic acid-->2-ETHOXY-MALONIC ACID DIETHYL ESTER-->2,4-Dihydroxypyrimidine-5-carboxylic acid-->4,6-Dihydroxy-2-methylpyrimidine-->5,7-BIS(TRIFLUOROMETHYL)-4-HYDROXYQUINOLINE-3-CARBOXYLIC ACID-->4-Chloro-7-(trifluoromethyl)quinoline-->ETHYL 4-CHLORO-6-(TRIFLUOROMETHYL)-3-QUINOLINECARBOXYLATE-->4-HYDROXY-5,7-BIS-TRIFLUOROMETHYL-QUINOLINE-3-CARBOXYLIC ACID ETHYL ESTER-->TRIETHYL 1,1,2-ETHANETRICARBOXYLATE-->ETHYL 2-(ETHYLTHIO)-4-HYDROXYPYRIMIDINE-5-CARBOXYLATE-->2,4,5-Trifluorophenylacetic acid-->Gliclazide-->2-MERCAPTOPYRIMIDINE-4,6-DIOL-->ETHYL 4-HYDROXY-6-(TRIFLUOROMETHYL)QUINOLINE-3-CARBOXYLATE-->Diethyl butylmalonate-->2-AMINODIETHYLMALONATE-->2-amino-6-chloropyrimidin-4(3H)-one-->3-CARBETHOXYUMBELIFERONE-->DIETHYL 2-(2-CYANOETHYL)MALONATE-->5-CARBETHOXYURACIL-->3-CARBETHOXY-2-PIPERIDONE-->2-Amino-6-hydroxypyrimidin-4(3H)-one ,97%-->Sulfamonomethoxine